Green Tech 101 : Is Hydrogen Safe?


In the face of escalating climate change, the need to mitigate global warming and reduce carbon dioxide emissions is rapidly increasing. Hydrogen, known for its remarkable energy density and zero emission is becoming a significant player in making this goal attainable.
As Hydrogen takes the news more often, public concerns are a natural response to new emerging technologies. But don’t worry, it's time we cleared the air of confusion and delve deeper into understanding hydrogen for what it really is — a beneficial and advancing energy carrier that is safe.
Hydrogen enables electricity generation goes beyond just powering vehicles, but homes and factories too. When we bring Hydrogen closer to our daily lives, it’s natural to think about its safety. In this article, we will clear up some misconceptions and discuss the efforts to take the safety of hydrogen technology to the next level.
Let’s Start by Busting Some Myths About Hydrogen!
What comes to mind when you first think of hydrogen? Skeptics associate it with explosive incidents like the infamous Hindenburg disaster or the powerful force of a hydrogen bomb. These impressions, while significant, can be misleading in terms of representing the potential power and safety of hydrogen energy in today's world.
According to a BBC Science Focus article, modern hydrogen power has no relation to the terrifying prospects of hydrogen bombs or catastrophic airship explosions. First, the Hindenburg airship’s fire source remains controversial, so it may not be the most accurate source to discuss hydrogen’s combustibility. An H-bomb or hydrogen explosion operates on the principle of nuclear fusion, the same process that fuels the sun. This process is only possible under extreme temperature and pressure levels, meticulously calculated to intentionally achieve just that, and would be safe to say your ordinary hydrogen-powered vehicle would never encounter.
Concerns may also arise regarding potential hazards at hydrogen fuel stations. The most well-known incident, which occurred by a fuel leak at a hydrogen fuel station in Norway in 2019, was due to an assembly error involving a particular plug in a high-pressure storage unit within a hydrogen tank. It's important to note that this issue was specific to this one filling station where the safety standards were not adhered to during installation.
In fact, the U.S. Department of Energy’s Hydrogen and Fuel Cells Technologies Office has stated that “a number of hydrogen’s properties make it safer to handle and use than the fuels commonly used today,” including gasoline, natural gas, uranium, jet fuel, and diesel. As such, given hydrogen's inherent characteristics, it stands as a viable solution, that has impressive credentials in terms of reducing risks related to explosions, fires, combustion, and even health.
Let's explore various reasons that underscore its nature.
1. Hydrogen is significantly lighter than air, reducing the likelihood of combustion.
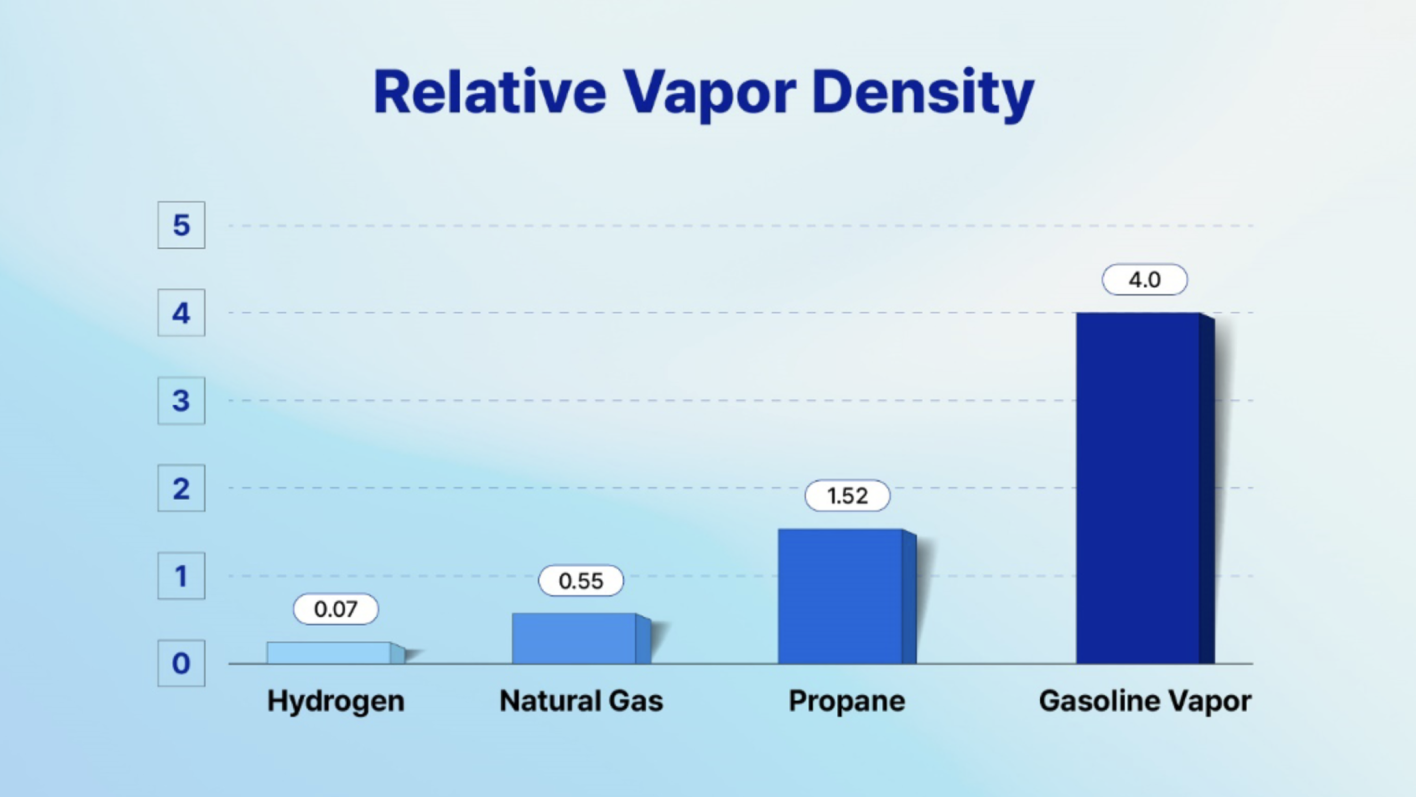
Hydrogen is a featherweight— approximately 57 times lighter than gasoline vapor and about 14 times lighter than air. Consequently, it minimizes its presence in an enclosed space where the density is increased. In simple terms, it means Hydrogen will likely occupy a smaller space in higher areas compared to other fuel vapor if present in a closed space. This characteristic makes hydrogen a comparatively safer option than other fuels if unintentionally released in a closed space.

2. Hydrogen is non-toxic and has a low asphyxiation risk.
According to U.S. Department of Energy, hydrogen is non-toxic and non-poisonous, and it doesn't contribute to soil contamination or water pollution, as it rises and disperse quickly when released. And while all gases can potentially cause asphyxiation when present in high concentrations, hydrogen's rapid dispersion makes the risk negligible. According to Medical News Today, prolonged and significant exposure to gasoline can lead to severe health problems, and consuming even a minuscule amount could potentially be lethal. However, not only the nature of hydrogen gas makes it challenging to inhale in large concentrations as it disperses too quickly, but the research from the National Institutes of Health indicates that inhaling high concentrations of up to 2.4% of hydrogen gas doesn't seem to result in notable negative effects in healthy adults. Some research even suggests it could alleviate certain symptoms.
3. Hydrogen's high ignition temperature reduces the risk of accidental ignition.
As stated by H2tools, the threshold at which hydrogen spontaneously ignites without the need for a flame or spark — known as the auto-ignition temperature — is considerably high, surpassing 575°C (1,067°F). This is significantly higher than the temperature required for other common fuels like diesel (345°C), natural gas (482°C) or gasoline (500°C) to ignite. This fact underscores the relative safety of hydrogen in standard conditions, as the likelihood of accidental ignition is lessened.


4. Hydrogen demands a higher oxygen concentration to cause an explosion.
We all know flame needs oxidizer like Oxygen to burn. More precisely, a concentration of at least 10% pure oxygen or 41% air. A tank filled only with hydrogen, with no oxygen, is safe from explosion.
Compared to fossil fuels, hydrogen requires a higher percentage of oxygen to ignite an explosion. The explosive range of hydrogen falls between 18.3% and 59% of oxygen. While this range seems broad, it is crucial to note that gasoline can ignite with just 1.1% to 3.3% of oxygen present . Furthermore, as described above, the chance of hydrogen exploding in open air is minimal due to its tendency to ascend and disperse rapidly.
The Safety of Hydrogen is More Than Just a Theory. It’s Proven.


Still worried about whether hydrogen is highly flammable? A 2001 experiment by Dr. Michael Swain with the University of Miami provides substantial insight. The study simulated two car fires — one caused by a punctured gasoline fuel line and the other by a faulty hydrogen connector. The gasoline-triggered fire destroyed the test vehicle, while the hydrogen fire ceased within two minutes, leaving the car nearly unscathed with an internal temperature of only 19.4°C (67°F) degrees.
Based on the 2001 experiment, it's indicated that a hydrogen-powered vehicle incorporates four separate safety mechanisms to maintain secure operation. With the passage of time, technological improvements in the safety of hydrogen vehicles have further reduced the minimal risks associated with them.
Hydrogen vehicles are equipped with multiple safety systems; even just one of them in operation can prevent undesirable incident, ensuring safety of the vehicle. For instance, there's the hydrogen sensor system, crafted to detect any hydrogen leaks, especially if there's an issue with the fuel line or the sealing of a component. Another key feature is the tank-mounted excess flow valve, which is a critical specification set to prevent abnormal leakage of hydrogen. Furthermore, there are flow-sensing computer programs that maintain the equilibrium between hydrogen flow and the fuel cell's consumption.
Thanks to these embedded safety measures, the risk of unforeseen incidents in hydrogen-powered vehicles is significantly minimized. As a result, such incidents are extremely rare, highlighting the dependability of these vehicles.
Hydrogen is Proven, and Hyundai is Making It Even Safer


Part 4: Hydrogen, Is It Safe? - YouTube (2:05-2:57)
While hydrogen already presents itself as a safe fuel, at Hyundai Motor Company, we've dared to ask, "Can we make it even safer?" For the past 25 years, Hyundai has been actively researching and developing hydrogen safety for automotive use, thoroughly assessing the risks associated with accidents and collisions. Hyundai's commitment to safety goes beyond merely meeting requirements – we have gone the extra mile to design even safer hydrogen tanks.
Hydrogen fuel tanks used in Hyundai vehicles are constructed from carbon fiber-reinforced plastic, a material ten times stronger than steel. These tanks are fortified with fireproof material on both the interior and exterior surfaces. Should they be exposed to flames, safety valves swiftly relieve internal pressure to ensure safety. In the event of a fire or crash, a Hyundai hydrogen tank, instead of exploding, would tear apart under extreme force, much like a thread. This leads to a rapid dispersion of hydrogen into the air, significantly minimizing potential hazards.
According to a report from OECD, should a crash and subsequent ignition occur in a hydrogen vehicle, it is highly unlikely that the hydrogen fuel will pose additional threat. When the hydrogen does encounter flames, it is predicted to produce a jet-like flame due to the activation of the pressure relief device reacting to a hydrocarbon fire. Also, as highlighted earlier, if a hydrogen storage tank ruptures and ignites, the hydrogen will quickly rise upwards and outwards, moving away from its structure, given hydrogen's buoyancy, its ability to spread, and its minuscule molecular size. In other words, creating a combustible scenario with hydrogen is notably difficult.
The safety of hydrogen extends beyond just a theory; it is proven through numerous tests and extensive research.
The opinions expressed herein include external journalists' personal opinions and do not represent the Hyundai Motor Company's view in any way.
------------------------------------------------------
- Kheir, John, Boston Children's Hospital “Safety of Inhaled Hydrogen Gas Mixtures in Healthy Volunteers.” Case Medical Research, 2019. https://doi.org/10.31525/ct1-nct04046211.
- Power Magazine. “Lessons Learned from a Hydrogen Explosion.” POWER Magazine, May 1, 2009. https://www.powermag.com/lessons-learned-from-a-hydrogen-explosion/.